
In such situations, all the aqueous ions will cancel out with each other, and no ion will be left behind. Bottom Lineĭon't forget that we cannot write a net ionic equation for a chemical reaction where all the products are aqueous.
#Chemical equation balancer net ionic how to
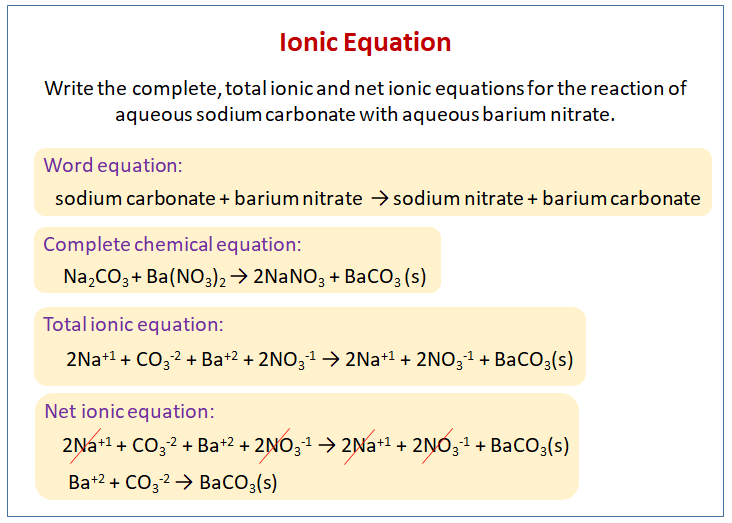
Related: How to calculate percent yield step by step. Thus, we cancel them out and write the final form of net ionic equation example as follow: Na + (aq) + Cl - (aq) + Ag + (aq) + NO - 3(aq) - > Na + (aq) + NO - 3(aq) + AgCl (s)Īs you can see, nitrate and sodium are present at both sides of the equation, thus they are spectator ions and not participating in the reaction. Now, break the electrolytes into ions such as First of all, you need to write the balanced chemical equation of this reaction which would be such as Suppose you have been writing net ionic equations for a reaction between silver nitrate and sodium chloride. Na + (aq) + Cl - (aq) + H + (aq) + OH - (aq)Īs you can see, only the H + and OH - are participating in the reaction while the Na + and Cl - keep on floating in the reaction medium from the very first point. Now break the electrolytes into ions such as Suppose a reaction between one mole of NaCl in one mole of HCl, the molecular chemical equation of this reaction would be such as Let's write a net ionic equation for a simple reaction to understand the procedure more precisely. Related: Also find how to determine oxidation number and oxidation state of elements? In the end, cancel out the spectator ions or eliminate them simply from the final net equation. All the species which would break into ions and possess an aq sign are called spectator ions only if they are present at both sides of the equation.All the species which are present either in solid, liquid, or gaseous form will not change.After writing the chemical symbol, charge, and ionic coefficient, write aq before each ion to show that the entire process has occurred in an aqueous medium.While writing the ions of each electrolyte, must mention its formula and charge along with the ionic coefficient (number of each ion to represent its quantity).



 0 kommentar(er)
0 kommentar(er)
